What Is Dissolved Water? | Lubripedia
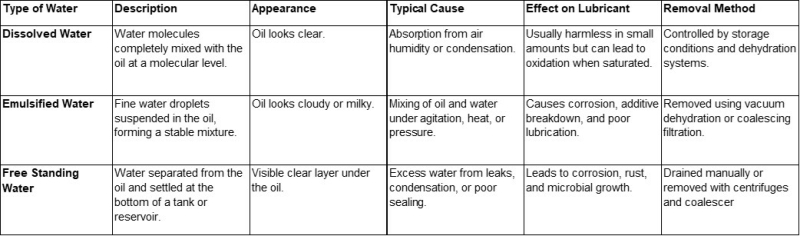
Dissolved water is water that is completely mixed with oil at the molecular level, forming a homogeneous solution.
Unlike free or emulsified water, dissolved water does not form visible droplets and does not make the oil appear cloudy or milky.
It is usually present in very small quantities and can remain undetected without proper testing.
How Dissolved Water Forms:
All lubricants and oils can hold a small amount of water naturally, depending on their type, temperature, and chemical composition.
Dissolved water forms when moisture from the air comes into contact with the oil and becomes absorbed.
Common causes include:
- Humidity in storage or operating environments
- Temperature fluctuations causing condensation
- Poorly sealed oil tanks or reservoirs
Exposure of lubricants to open air during handling
At this stage, the oil may appear clear even though water is present at a molecular level.
When Dissolved Water Becomes a Problem:
While small amounts of dissolved water are usually harmless, excess moisture can cause the oil to become saturated.
Once the saturation point is reached, any additional water will start to form emulsions or free water, leading to:
- Reduced lubrication performance
- Corrosion of metal components
- Breakdown of additives and base oil
Formation of sludge and oxidation products
High dissolved water levels can also reduce dielectric strength in transformer and turbine oils.
How to Control Dissolved Water:
- Store lubricants in sealed containers with desiccant breathers.
- Keep oil reservoirs closed and protected from humidity.
- Use vacuum dehydration or dry air systems for critical applications.
Perform regular oil analysis to measure water content (using Karl Fischer titration or similar methods).
Maintaining low water content helps extend oil life and ensures reliable equipment operation.
Comparison table
See also:
- What is Emulsified Water ?
- What is Free Water?
- What is Water Contamination?
- What is Oil Analysis?
- What are Oil Contamination Problems?
